- Home Page
- Company Profile
-
Our Products
- Pharmaceutical Capsule
- Capsula de Gelatina Blanda de Nintedanib 100mg
- Trientina Clorhidrato de Capsula 250 MG
- Temozolomide Capsule
- Tacrolimus Capsule
- Lenalidomide 10mg Capsule
- Etoposide Capsules
- Oseltamivir Capsula
- Pomalidomide Capsule
- Nintedanib Gelatina Blanda Capsula
- Dimethyl Fumarate Delayed Release Capsule 120 MG
- Molnupiravir Capsule
- Ibrutinib Capsule
- Enzalutamide Capsule
- Pomalidomide Capsules
- Ibrutinib Capsules
- Temozolomide Capsule
- Enzalutamide 80mg Capsule
- Tetracycline (500mg) Capsule
- Tacrolimus (1mg) Capsule
- Pharmaceutical Injection
- Denosumab Solution For Injection
- Bevacizumab Injection
- Polymyxin B For Injection
- Recombinant Human Parathyroid Injection 750mcg/3ml
- Cefotaxime Sodium
- Doxorubicin Injection Solution
- Pemetrexed Injection
- Ulinastatin Injection
- Paclitaxel Injection
- Docetaxel Concentrated Injection
- Ferric Carboxymaltose (50mg/ml)
- Bortezomib Injections
- Bleomycin Injection
- Atracurium Besylate
- Rituximab Injection
- Ifosfamide Injection
- Infusion Solucion Concentrada Polvo De Trabectdin
- Lactato De Milrinona Solucion
- Phospholipids Injection
- Zoledronic Acid
- Colistimetato Solucion
- 250 Mg Antibody Protein Injection
- Zoledronic Injection
- Liposomal Amphotericin B
- Vecuronium Bromide
- Cisatracurium Besylate
- Eritropoyetina Humana Recombinante Solucion
- Gemcitabine Injection
- Estreptoquinasa Recombinante Para Solucion
- Equine Rabies Immunoglobulin (1500iu)
- Etanercept Inyectable Solucion 50 MG
- Bcg Para Inmunoterapia Ip
- Hucog 5000 HP
- Paclitaxel Suspension Inyectable 100mg
- Denosumab (60mg) Injection
- Mitomycin injection
- Irinotecan (20mg) Injection
- Eribulin Mesylate
- Pegilado Alfa 2b Para Solucion
- Pegfilgrastim Injection
- Doxorubicin Injection (50mg)
- Azacitidine Injection
- Ramucirumab Injections
- Asparaginase Injection
- Casirivimab Imdevimab
- Paclitaxel Injection 100mg
- Dacarbazine Injection
- Cyclophosphamide Injection
- Eribulin Mesylate 0.5mg
- Tenecteplase (40mg)
- Ranibizumab Solution for Injection
- Rocuronium Bromide
- Ferric Carboxymaltose
- Hyaluronic Acid
- Doripenem Injection
- Busulfan Injection
- Pharmaceutical Syrup
- Pharmaceutical Tablet
- Efavirenz Tablets
- Sofosbuvir Tablet 400MG
- Capecitabine Tablets
- Viraday Tablet
- Mycophenolate Mofetil
- Daclatasvir y Sofosbuvir Tableta
- Darunavir Tablets
- Sofosbuvir Tablets 400 mg
- Emtricitabine And Tenofovir Disoproxil Fumarate Tablets
- Everolimus Tablets 0.5mg
- Tofacitinib (5mg) Tablets
- Abiraterone Acetate
- Bosentas 62.5 Tablets
- Tenofovir Disoproxil Fumarate Tablet
- Tenofovir Alafenamide
- Dolutegravir Tablets
- Lopinavir and Ritonavir Tablets
- Amoxicilina Clavulanato De Potasio Tableta
- Abacavir Tablets
- 400 MG Imatinib Mesylate Tablet
- Abacavir Sulphate
- Mycophenolate Sodium Tablet
- Dasatinib Tablet
- Axitinib Tablet
- Maraviroc Tablet
- Exemestane Tablets
- Molnupiravir Tablet
- Capecitabine Tablet
- Cefixime Tablets
- Midodrine Tablets
- Capecitabine 500 Tablet
- Azithromycin (250mg) Tablet
- Semaglutide Tablets
- Molnupiravir Capsules 200mg
- Maraviroc 150 mg Tablets
- Forstavir L Tablet
- Diltra 30s Tablets
- Hidroxicloroquina Sulfato
- Nicoumalone Tablets
- Injectable Solution
- Anticancer Injection
- Anticancer Tablets
- Cabozantinib Tablets 60 mg
- Rucaparib Tablets 200mg
- Cabozantinib Tablets 20mg
- Cabozantinib Tablet
- Lenalidomide Capsule
- Pazopanib Tablet
- Lapatinib Tablets
- Sorafenib Tablets
- Pazopanib Tablets 400mg
- Abemaciclib 200mg
- Cabozantinib Tablets 40 mg
- Nintedanib Capsules 150mg
- Abiraterone Acetate 500 mg
- Etoposide Capsules IP 50mg
- Everolimus Tablet
- Afatinib Tablets
- Oral Suspension
- Pharmaceutical Capsule
- Contact Us
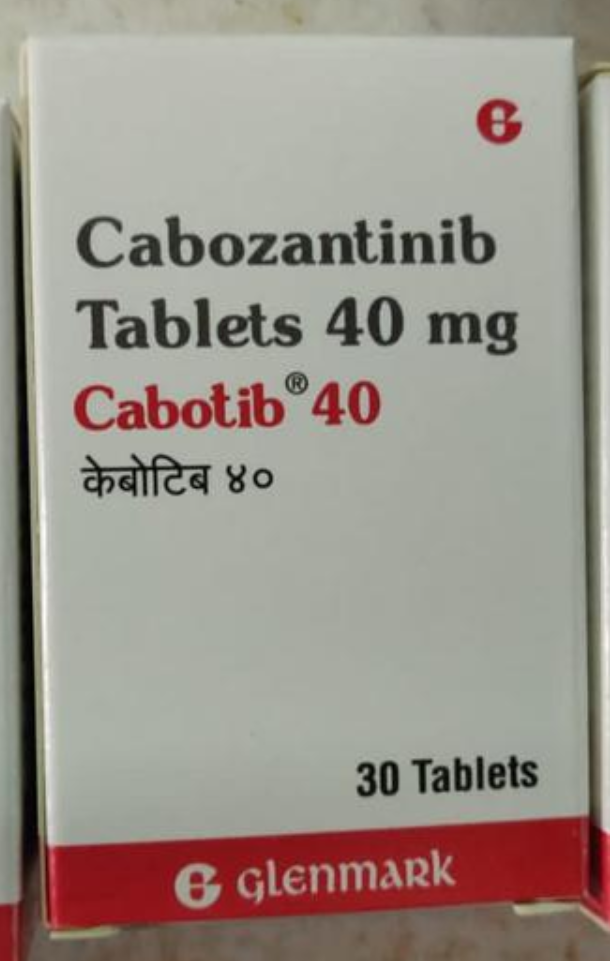
Cabozantinib Tablet
121187.0 INR/Bottle
Product Details:
- Indication Anti-cancer
- Dosage Form Tablets
- Salt Composition Cabozantinib
- Origin India
- Fermentation Smell Normal Smell
- Storage Instructions Cool and dry place
- Shelf Life 12 Months
- Click to View more
X
Cabozantinib Tablet Price And Quantity
- 121187.0 INR/Bottle
- 1 Bottle
Cabozantinib Tablet Product Specifications
- India
- Cabozantinib
- Cool and dry place
- Normal Smell
- 12 Months
- Tablets
- Anti-cancer
Cabozantinib Tablet Trade Information
- 12 Days
- Asia, South America, Eastern Europe, Western Europe
Product Description
Renal cell carcinomaCabozantinib is indicated as monotherapy for advanced renal cell carcinoma as first-line treatment of adult patients with intermediate or poor risk in adults following prior vascular endothelial growth factor (VEGF)-targeted therapyCABOZANTINIB TABLETS is sold internationally as REVLIMID. It's also available in India other brand such as CAZANAT from NATCO, CABOZANIB from BDR PHARMACUTICAL, CABOTRES from CIPLA, CABDUAL from INTAS.
FAQs of Cabozantinib Tablet:
Q: What is the indication for Cabozantinib Tablet?
A: Cabozantinib Tablet is indicated as an anti-cancer medication.Q: What is the salt composition of this product?
A: The salt composition of this product is Cabozantinib.Q: How should Cabozantinib Tablets be stored?
A: Cabozantinib Tablets should be stored in a cool and dry place.Q: What is the shelf life of Cabozantinib Tablets?
A: The shelf life of Cabozantinib Tablets is 12 months.Q: Does Cabozantinib Tablet have a fermentation smell?
A: No, Cabozantinib Tablet has a normal smell.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Anticancer Tablets' category
We are mainly deals in Foreign Countries.

 Call Me Free
Call Me Free